
Naomi Walsh Cancer Research Group Establish New Ways to Study Pancreatic Cancer
A new publication from researchers of the School of Biotechnology/National Institute for Cellular Biotechnology has detailed a novel means of effectively modelling pancreatic cancer in the laboratory.
Pancreatic cancer is one of the deadliest forms of cancer with an approximate survival rate of 9%. The rapid progression of the disease, in addition to the aforementioned high mortality rate, make it an incredibly difficult condition to research. On its current trajectory, by 2030 it is predicted to surpass both breast and colorectal cancer to become the second most common cause of cancer-related death in the United States.
Consequently, there is a clinical unmet need for reliable, tractable cellular models which enable the preclinical research required to expedite the validation of functional genetic variants in pancreatic cancer development and the establishment of effective treatment strategies towards this disease – a challenge which has been met by way of the exciting new approach led by Assistant Prof. Naomi Walsh in collaboration with colleagues from the National Institute for Cellular Biotechnology (NICB) at DCU, and international collaborators from Denmark.
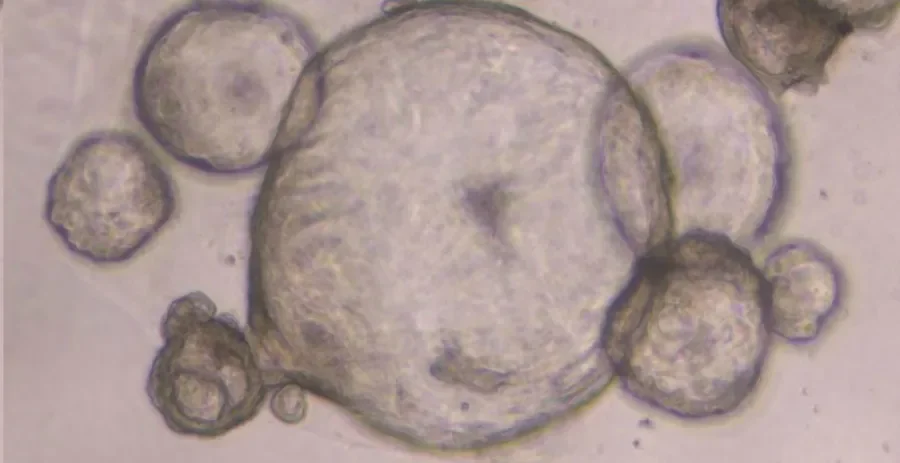
The work, supported by Science Foundation Ireland, focusses on the development and validation of organoids (or miniature tumours) as a more physiologically relevant model to study pancreatic cancer.
‘Traditionally two-dimensional cell culture has been the model of choice for researchers in the field of therapeutics design’, explains Assistant Prof. Naomi Walsh, ‘their importance in cancer research is indisputable, however they present a number of limitations which has in certain instances curtailed and even stalled therapeutic development and testing’.
‘These limitations not only impact on the greater clinical populations but also instances of rare genetic forms of this disease. For example, some forms of cancer require a precision medicine approach whereby an individual may respond better to a personalised cancer treatment. The traditional two-dimensional cell culture approach, owing to these limitations, often does not maximise the potential of samples obtained from individuals for this purpose’.
‘We have developed a novel means of generating three-dimensional organoids using patient samples which capitalises on this potential’, describes Shannon Nelson, the lead author of the publication, ‘in culturing the cells in a similar environment to that of the body, they behave in a more representative form of the disease. We have found our “cell line organoids” or “CLOs” accurately capture the pancreatic disease state in comparison to traditional 2D methods as demonstrated in our examination of cell morphology, specific stem cell markers, and transcriptomic signatures across existing methods’.
‘We anticipate this approach represents an exciting alternative to existing methodologies and offers its own distinct advantages. Using it for genetic validation and high throughput screening will ultimately expedite our understanding and characterisation of this disease, in turn reducing the time taken to develop more effective therapeutic interventions against such’.
For more information, the group’s latest publication can be viewed here.
Also, for those interested in the area outside of this publication, the Naomi Walsh Cancer Research Group have also recently published a review on the topic which can be found here.
